Alzheimer’s disease (AD) can develop over the course of many years without obvious symptoms until it has become quite advanced and is potentially beyond the point of reversal. The research states that the root cause of Alzheimer’s has yet to be discovered; however, if we continue to look for that ‘one thing,’ we may never find it. The development of Alzheimer’s disease is likely due to several factors that contribute to neuronal degeneration over several years.
The characteristic markers of AD are the build-up of beta amyloid plaques and the formation of neurofibrillary tangles (NFTs) within the brain. While we know these markers are associated with neuronal degeneration and brain deterioration, what allows for them to develop? Are they a cause, effect, or both? The prevention of chronic degenerative diseases such as cardiovascular disease (CVD), hypertension, diabetes, cerebrovascular disease, and drivers of chronic inflammation have proven beneficial when applied to the prevention of AD.
In this article, we will explore some of the risk factors associated with the development of AD and their association with cardiovascular disease and chronic inflammation. Though the literature states there is no common causal link between CVD and AD, there is strong evidence that CVD can contribute to the progression of AD as they share many common risk factors. There are also many drivers of chronic inflammation that increase the risk of developing AD. If we can address the common and familiar risk factors associated with CVD and inflammation, we have the potential to reduce the risk of developing AD.
Risk factors for Alzheimer’s
Age, family history, genetics, cardiovascular disease (CVD), and related disorders such as diabetes, hypertension, high cholesterol, and obesity can contribute to the development of Alzheimer’s disease. Other risk factors include traumatic brain injury, periodontal disease, gut dysbiosis, chronic infections, smoking, excessive alcohol consumption, depression, social isolation, and a sedentary lifestyle (1,2). Suboptimal levels of nutrients, chronic inflammation, reduced hormones, loss of neurotransmitters, mitochondrial dysfunction, reduced brain glucose metabolism, and toxic exposures can also contribute to the development of AD and other chronic degenerative disorders (3).
Cardiovascular disease and inflammation strongly associated with Alzheimer’s disease
As mentioned above, there are numerous potential contributors to the development of Alzheimer’s disease. Some of these contributors are underlying disease processes and some are due to environmental exposures, deficiency states, infections, and cellular and metabolic dysfunction. Because this is a blog and not a book, examining the development of Alzheimer’s from a few key risk factors may offer a simplified perspective.
Cardiovascular disease and inflammation contribute to and are a consequence of many of the underlying processes associated with the development of AD. Despite numerous research studies over the past several decades, no singular cause of AD has been established. From a simplistic perspective, AD may be the brain’s response to inflammation and the effects of vascular disease. Perhaps by analyzing and addressing the risk factors associated with CVD and inflammation, we can reduce the occurrence of AD.
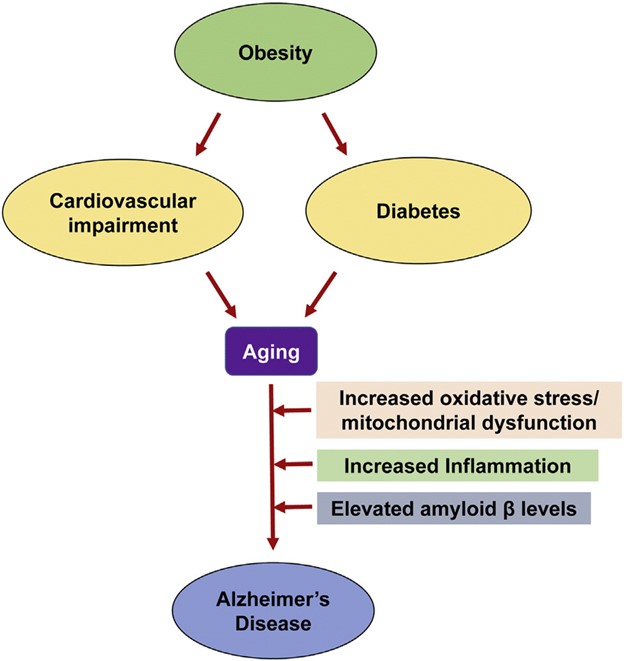
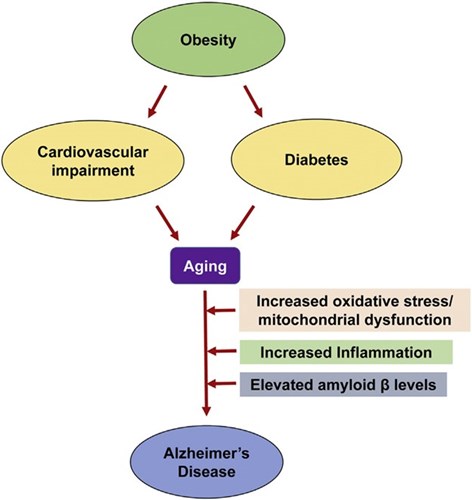
Fig. 1. Common pathways of aging-associated disorders: The risk of Alzheimer's disease (AD) in elderly individuals is increased by other aging-associated comorbidities including obesity, diabetes and cardiovascular impairment. Oxidative stress, mitochondrial dysfunction and chronic inflammation observed in these conditions are also some of the important causes of AD.
Image Credit: Common Neurodegenerative Pathways in Obesity, Diabetes, and Alzheimer’s Disease - Scientific Figure on ResearchGate.
Cardiovascular disease and AD
According to the Alzheimer’s Association, there is an 80% correlation between the development of AD and the presence of cardiovascular disease. Anything that has the potential to damage the heart and blood vessels can also damage the brain. Also noted by the Alzheimer’s Association, the development of beta-amyloid plaques and NFTs may not always lead to AD. Autopsy studies suggest that plaques and NFTs may be present in the brain without causing symptoms of cognitive decline unless the brain also shows evidence of vascular disease (4).
Cardiovascular diseases are a diverse set of disorders that include atherosclerosis, arteriosclerosis, and cerebrovascular disease. Although various therapies have been developed to treat several contributors to CVD, pathological alterations are often irreversible and cannot be completely cured (5). Conditions associated with the development of CVD tend to emerge in midlife, so it is imperative that measures be taken early to prevent advancement of this common risk factor for AD.
The risks of developing AD and cardiovascular disease are both increased by a common range of conditions that include hypertension, dyslipidemia, high cholesterol, diabetes, obesity, gut dysbiosis and periodontal disease. Lifestyle factors that contribute to these conditions include smoking, physical inactivity, excess alcohol consumption, and nutritional deficiencies.
Many of these conditions occur together and contribute to the overall disease process that leads to CVD and AD. Let’s take a closer look at a few of these conditions.
Hypertension
Hypertension adversely affects the structural integrity of cerebral blood vessels, promotes the formation of atherosclerotic plaques in cerebral arteries, and induces hyperlipidemia (5). In a double-blind placebo-controlled study, the use of antihypertensive medication reduced the risk of dementia by 50% as compared to the control group. It is postulated that blood pressure regulation in midlife may postpone the onset of AD. However, the use of antihypertensives in those with existing AD showed no improvement in cognitive performance (5). Perhaps this was a ‘too little, too late’ scenario. If one has the diagnosis of AD, the process of disease development has been occurring for several years and addressing a potential risk factor ten or more years later cannot undo the damage.
Cerebrovascular disease
Cerebrovascular disease is a common feature of AD and other forms of dementia. The presence of beta amyloid can be a cause and consequence of cerebrovascular disorders. Reduced blood flow to the brain and disruption of the blood-brain barrier can be induced by the presence of beta amyloid and vascular pathology. These conditions can lead to lower oxygen supply resulting in acidosis, mitochondrial dysfunction, and oxidative stress. Consequently, this process further leads to the production of reactive oxygen species and perpetuates the formation of beta amyloid and NFTs. Disruption of the blood-brain barrier (BBB) impairs the clearance of beta amyloid from the brain and cerebrospinal fluid. Decreased blood flow and disruption of the BBB both lead to an accumulation of beta amyloid and the formation of NFTs resulting in neuronal loss and cognitive decline (5).
Type II diabetes
According to several epidemiological studies, type 2 diabetes (T2DM) doubles the risk for AD as well as vascular dementia. Patients with T2DM were more likely to have cerebral infarcts and more extensive vascular pathology leading to a higher risk of dementia. Interestingly, the brains of AD patients showed reduced insulin production and reduced insulin receptors. Impaired glucose metabolism in the brain is one of the most recognized abnormalities in AD (5).
Insulin affects the electrochemical and biochemical action on neurons that form neurotransmitters associated with memory and learning. Insulin also influences the enzymes that breakdown beta amyloid. A reduced ability to respond to insulin signaling is directly related to the development of neuroinflammation, amyloidogenesis, oxidative stress, and mitochondrial dysfunction. Disruption of the insulin-signaling pathway is commonly referred to as brain insulin resistance or “type 3 diabetes” and ultimately results in impairments in synaptic, metabolic, and immune response functions in the brain (5).
Gut microbiota
An imbalanced gut microbiota may play a secondary role in the pathophysiology of various diseases due to its influence on immune function and inflammatory processes. The gut microbiome of those with CVD produces more proinflammatory mediators enhancing chronic inflammation and impeding gut barrier function. Impaired gut barrier function allows for the translocation of microbial metabolites such as lipopolysaccharide (LPS) which has been associated with advancement of CVD and poor glycemic control (5).
The interaction between the central nervous system and the gut is well established and commonly referred to as the gut-brain axis indicating bi-directional communication between the gut and the brain. Both the BBB and the intestinal mucosa are more permeable during an inflammatory state. Increased permeability allows for the passage of LPS, a known neurotoxin, from the gut into general circulation inciting an inflammatory cascade which may potentiate neuroinflammation and neuronal impairment. LPS can stimulate the misfolding of beta-amyloid proteins and activate the inflammasome pathways perpetuating neuronal damage, chronic inflammation, and oxidative stress (5).
Periodontal disease
Periodontal disease is a known contributor to CVD. The bacteria associated with periodontal disease, (P. gingivalis, T. denticola, T. forsythia) have also been identified in the brains of those with AD. As mentioned above, the BBB becomes more permeable during an inflammatory state. The proximity of the oral cavity to the brain may allow for easy translocation of oral bacteria to the brain under a state of inflammation. Beta-amyloid is an antimicrobial peptide that increases in the presence of infections. It has been postulated that one of the drivers of accumulated beta-amyloid plaques is the colocalization of these plaques at the site of infections and inflammation in the brain (5).
Inflammation
Commonly associated with an immune response to infection or injury, inflammation is a normal process that attacks an infection and promotes a healing response. Many of the risk factors associated with the development of AD can promote chronic inflammation within the brain. Sustained inflammation has emerged as a core driver of the formation of beta-amyloid plaques and neurofibrillary tangles (NFTs). The presence of the beta-amyloid and NFTs results in persistent activation of microglia, the brain’s resident macrophages, which has further been implicated in the pathogenesis of AD. This sustained inflammatory response has been observed in the postmortem brain tissue of AD patients (6).
Acute inflammation in the brain occurs in defense against infections, toxins, and injury; however, an imbalance between pro-inflammatory and anti-inflammatory signaling results in chronic inflammation that sustains activation of microglial cells and the release of various cytokines that mediate inflammation. Markers of chronic brain inflammation are a common feature of neurodegenerative disorders and also occur in Parkinson’s disease, traumatic brain injury with chronic encephalopathy, amyotrophic lateral sclerosis, and multiple sclerosis (6).
Inflammation also occurs in response to the pathological changes in the brain related to beta-amyloid and NFTs but also perpetuates and exacerbates the development of these two core pathologies. Brain inflammation begins as a neuroprotective response in the acute phase but is detrimental when it becomes chronic. Chronically activated microglia release proinflammatory and toxic products including reactive oxygen species, nitric oxide, and various cytokines (6).
It is suspected that the microglia are primarily activated by the presence of beta-amyloid and are initially effective at clearing beta-amyloid plaque; however, microglial capacity for removing amyloid plaque diminishes while its capacity for producing inflammatory cytokines is sustained. Proinflammatory cytokines also trigger a signaling response that results in the hyperphosphorylation of tau contributing to the development of neurofibrillary tangles (NFTs) adding to the inflammatory and neurodegenerative process. This results in a feed forward loop that leads to reactive microgliosis and sustained brain inflammation (6).
Beta-amyloid protein: cause or effect?
Though vilified as the causative agent of AD, beta-amyloid protects the brain from a broad spectrum of infectious agents, supports the integrity of the BBB, enhances recovery from traumatic brain injury, reduces oxidative stress, and boosts synaptic plasticity, learning and memory. In order for beta-amyloid to exert its positive effects, it must exist in a state of hormesis whereby low-dose amounts have a beneficial effect and high-dose amounts are either inhibitory to optimal function or toxic. The presence of beta-amyloid plaques in the brain of AD patients is indicative of the brain’s attempt to heal itself in the presence of underlying dysfunction often driven by the many risk factors listed above. Getting rid of the beta-amyloid plaques does not remove what drives the overproduction (7).
Early intervention
While we cannot alter our genetics or family history, we can proactively take measures to optimize health by addressing the risk factors for cardiovascular disease and reducing triggers for chronic inflammation. We can protect the brain with a nutrient-dense diet rich in anti-oxidants, and engage in regular physical activity, mental stimulation, and social interaction. Avoiding toxic exposures through food and our environment, supporting the gut and oral microbiome, and enhancing a healthy immune response, reduces oxidative stress and inflammation that drive the overproduction of beta-amyloid and NFTs.
Mid-life is when the pathological changes that lead to AD begin to develop, so it is critical to implement dietary and lifestyle changes early enough to potentially alter the trajectory of AD and other chronic degenerative diseases that may contribute to its development. All of the actions we can take to reduce our chances of developing AD also contribute to good health on multiple levels and most of these recommendations are familiar to all of us.
Regular screening for risk factors associated with the development of AD can direct efforts to make positive changes that may have lasting effects for the prevention of many chronic degenerative diseases. ZRT Laboratory offers a variety of metabolic tests that measure risk factors associated with CVD, diabetes, and inflammation. Additionally, ZRT offers comprehensive evaluation of urinary neurotransmitters, sex hormones, heavy metals, cortisol and thyroid hormones – all of which influence brain health and cognitive function at any age.
Early and consistent interventions can change the trajectory of disease processes while monitoring progress through objective data can further guide our efforts along the way. When we consider that Alzheimer’s disease, as of this writing, has no effective cure, prevention of common risk factors is our best bet.
References:
- “Alzheimer’s Disease - Causes.” Nhs.Uk, 10 May 2018.
- Omura, John D. “Modifiable Risk Factors for Alzheimer Disease and Related Dementias Among Adults Aged ≥45 Years — United States, 2019.” MMWR. Morbidity and Mortality Weekly Report, vol. 71, 2022.
- Bredesen, Dale E. “Reversal of Cognitive Decline: A Novel Therapeutic Program.” Aging (Albany NY), vol. 6, no. 9, Sept. 2014, pp. 707–17. PubMed Central.
- Alzheimer's Association - Vascular Dementia
- Leszek, Jerzy, et al. “The Links between Cardiovascular Diseases and Alzheimer’s Disease.” Current Neuropharmacology, vol. 19, no. 2, Feb. 2021, pp. 152–69. PubMed Central.
- Kinney, Jefferson W., et al. “Inflammation as a Central Mechanism in Alzheimer’s Disease.” Alzheimer’s & Dementia: Translational Research & Clinical Interventions, vol. 4, Sept. 2018, pp. 575–90. PubMed Central.
- Morley, John E., et al. “What Is the Physiological Function of Amyloid-Beta Protein?” The Journal of Nutrition, Health & Aging, vol. 23, no. 3, Mar. 2019, pp. 225–26.