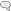ZRT Laboratory has been working with dried samples for over 15 years, and the number of tests we offer using dried urine and blood spot make us the world expert on dried sample validation and commercialization. ZRT Laboratory specializes in dried sample types because of their superior stability, ease of collection, simple shipment and reduced storage space. Dried urine and blood spot collection is popular with researchers and remote clients, as these sample types open the door for testing in locations that lack refrigeration and equipment necessary for liquid sample collection.
In order to offer clinical testing using dried samples, ZRT Laboratory must go through a rigorous validation process for each analyte. If validation is successful and the test is commercialized, it is considered a Laboratory Developed Test (LDT). LDTs are overseen by the Centers for Medicare and Medicaid (CMS)/Clinical Laboratory Improvement Amendments (CLIA) and the Food and Drug Administration (FDA), and can only be performed at the laboratory completing the validation.
The following is a real example of the test validation process for an LDT – in this case the dried urine rare elements assay (gadolinium [Gd], thallium [Tl], uranium [U], and creatinine) run by Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
Clinical Usefulness
The first thing considered before a test is developed and validated is its clinical usefulness. It is important to choose the appropriate sample type and be able to explain results. This is usually a multi-week process of researching scientific literature while determining routes of excretion and expected concentrations.
Method Development
The method development process can be simple or complex depending on whether there is an established liquid method as a starting point. Multiple filter paper lots need to be screened to check for consistency, and extraction efficiency needs to be tested. Some methods require a concentrated sample for maximum sensitivity while others need further sample dilution, so sample requirements and extraction volume must be determined. The dried urine rare elements assay, for example, must be able to test down in the low parts-per-trillion (ppt), so a concentrated sample is needed.
Preparing (Drying) Samples
All samples used during the validation process, which include standards, calibrators, controls, spikes, patient samples, blanks, and proficiency samples, are dried on laboratory grade filter paper and stored in a -20°C freezer. Some samples are left at room temperature and others refrigerated or heated for stability testing under different storage conditions, which is a part of the validation.
Accuracy
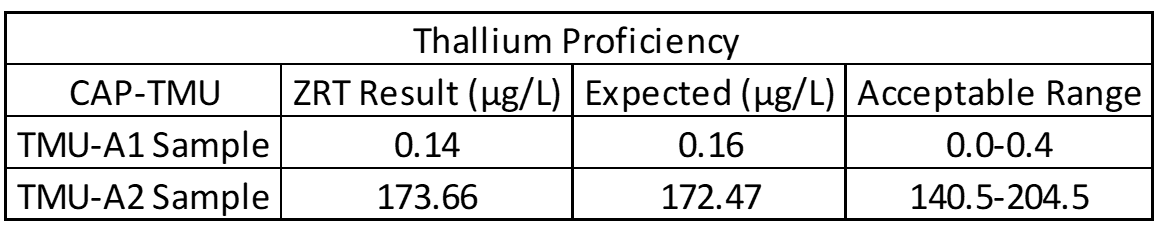
Accuracy testing is the most important part of the validation process. This covers extraction efficiency of sample from filter paper and verifies that standards and calibrators are performing properly. Accuracy also ensures that results obtained at different laboratories are comparable. For this purpose, external proficiency samples are tested, if available. In the case of the rare elements assay, thallium proficiency samples are available through the College of American Pathologists Trace Metals Urine Program (CAP-TMU). Liquid proficiency samples are dried on filter paper, run as a “patient sample,” and compared to expected values when results are available. Split samples are also sent to reference laboratories for comparison.
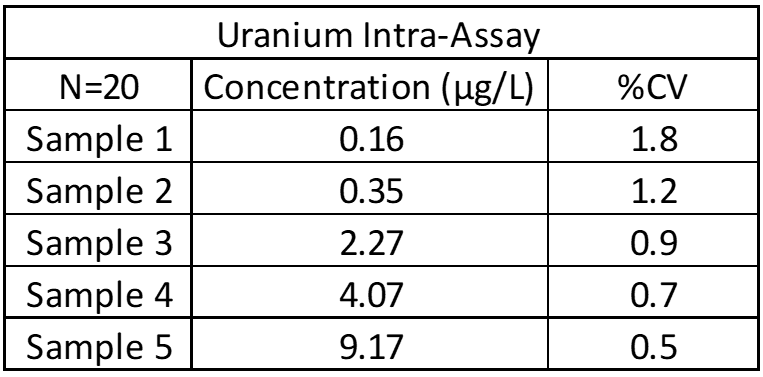
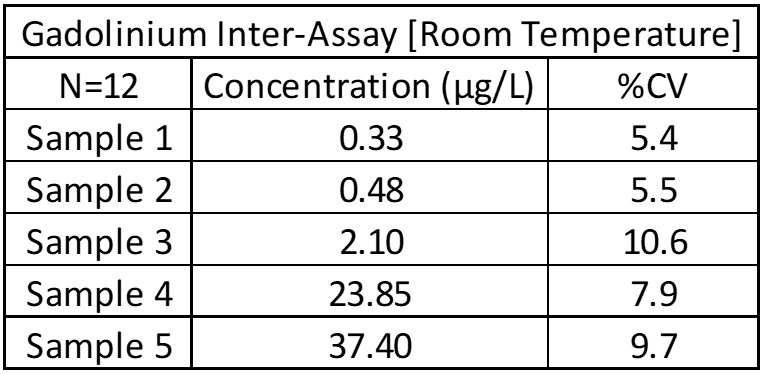
Inter-Assay and Intra-Assay Precision
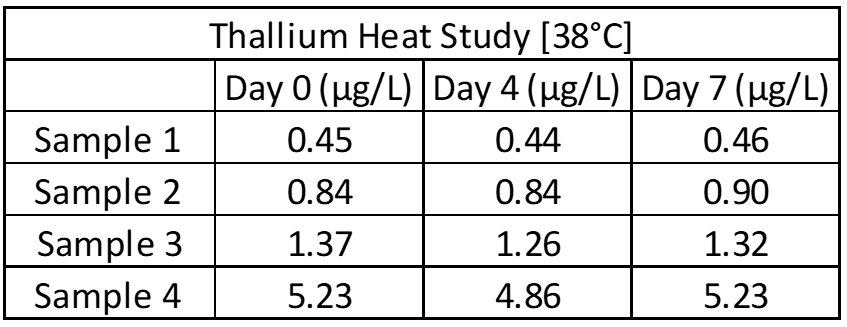
Inter- and intra-assay precision looks at the amount of variation between identical samples run on a single day and over a month period (10+ replicates of each). The coefficient of variation (%CV = Standard Deviation/Average X 100) is used to judge assay variation, with <20% considered acceptable. Inter-assay precision includes samples kept at room temperature, refrigerated and frozen to test for stability under all conditions. Heated samples at 38°C (100°F) are also tested in a week-long study to replicate samples being left in a hot area such as a mail van.
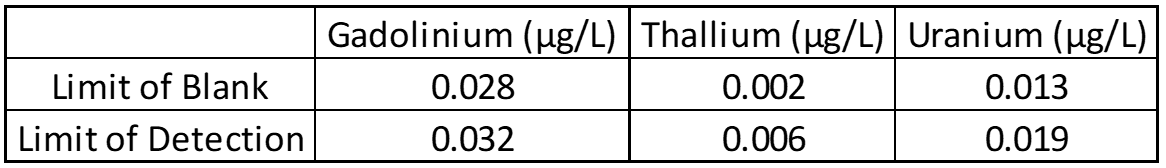
Limit of Blank, Detection and Quantification
The limit of blank (LOB), limit of detection (LOD), and limit of quantification (LOQ) help determine the smallest amount of analyte that can reliably be detected. Twenty blank and low concentration samples are used to determine LOB and LOD, while LOQ is based on other parts of validation like linearity. In most cases LOD and LOQ are identical, but this must be confirmed.
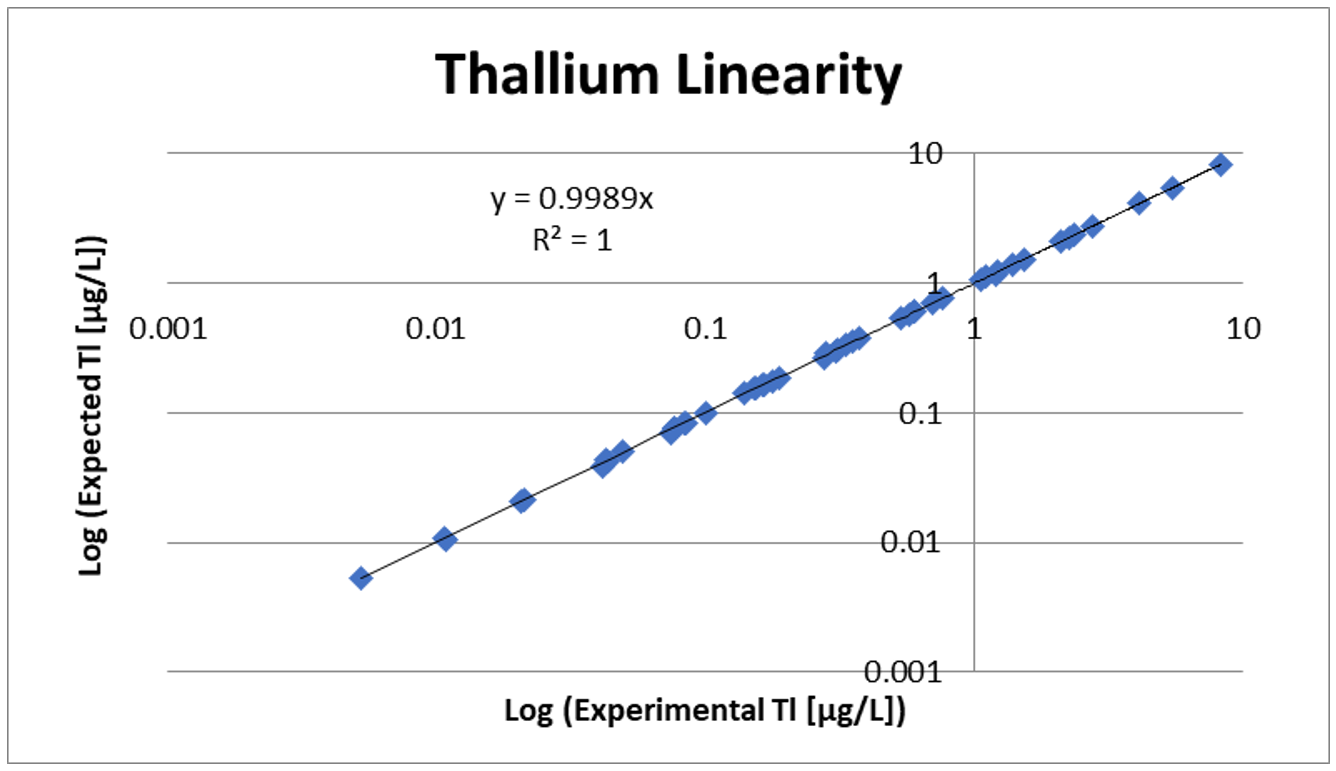
Linearity and Spike Recovery
Linearity testing involves diluting samples using extracted blank to make sure the low, mid, and high ranges of the assay are acceptable. Linearity also confirms that the assay is linear down to the LOD. Spike recovery is performed to show that when a known amount of analyte is added to a sample, the assay result increases by that set amount.
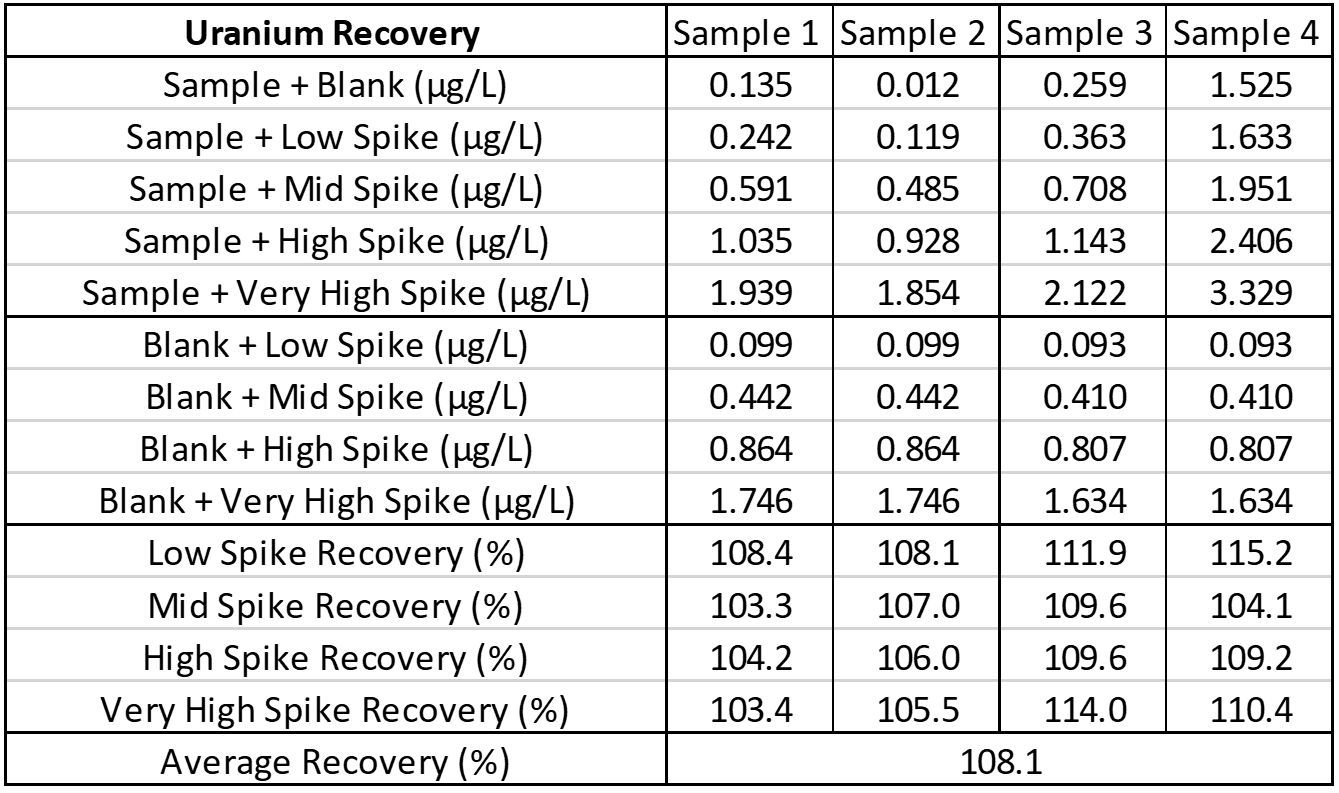
Interference
Interference can be tested in different ways. For hormone assays you may look at interferences from hormones with a similar structure, but for ICP-MS a potentially interfering element is spiked into a sample, then blank is spiked into a second identical sample, and results run and compared. The following is an example of barium interference on gadolinium and what an interference-free run looks like for thallium.
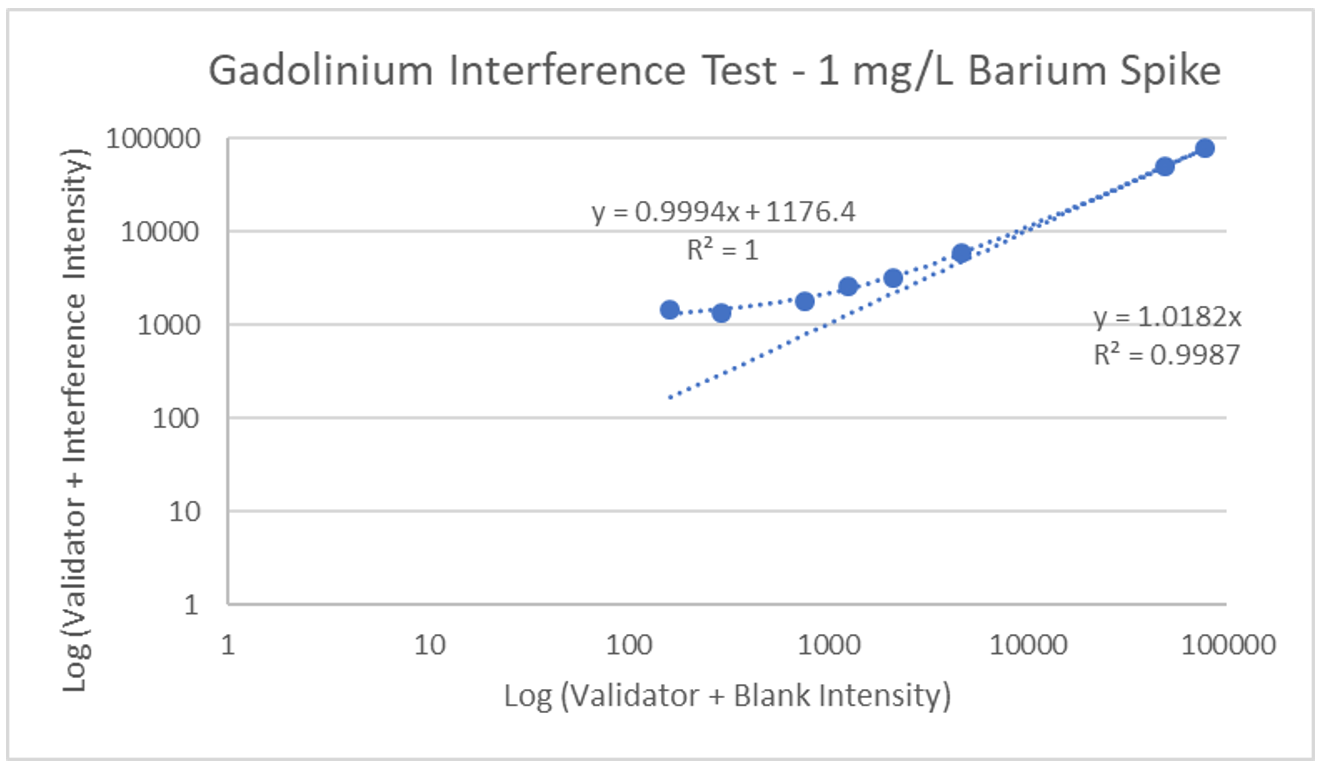
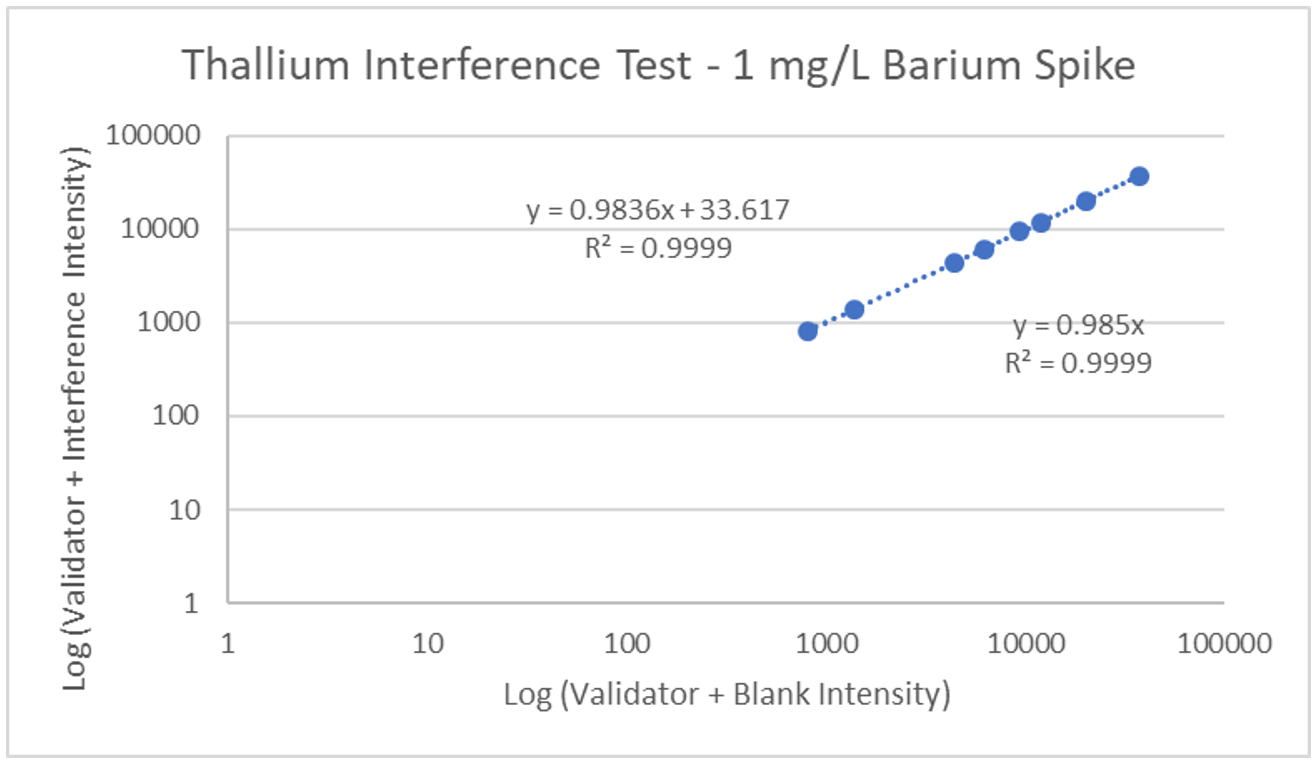
Explanation: Gadolinium155[14.8% abundance] has a known interference from Barium Oxide at mass 155 (Barium138[71% abundance]+Oxygen17[0.038% abundance]). The concentration of barium tested was 1 mg/L. The geometric mean barium level measured in the U.S. general population aged 6 and older is reported by the CDC as 1.56 µg/g creatinine. This level is >600x higher than that used in the spike solution. Barium is therefore not expected to be a problem in gadolinium analysis.
Reference Range
Each laboratory must develop their own analyte reference range that ideally represents a healthy testing population. At ZRT Laboratory we de-identify patient samples from prior testing or set up a collection study to obtain samples, and typically run 500+ samples to establish ranges. Generally, a 5th-95th percentile is used, but it is dependent on the analyte and literature. A 5th-95th percentile means that ideally 5% of people testing will be low and 5% will be high. This is meant to detect outliers, not necessarily deficiency or toxicity. The rare elements gadolinium, thallium, and uranium are non-essential elements, so the reference range is set at >95%, which includes creatinine correction to adjust for hydration of the test subject.

Wrapping up Validation
There are many other small tests that are necessary to complete validation. These include lab technician comparisons, filter paper lot variation and background interference, creatinine correction studies, sources of contamination and an acidified filter paper comparison (since acidified filter paper is used for some ZRT testing). The entire process may take as little as 3 months to as long as years and may need to be restarted from scratch if a step fails.
Future Developments
We continue to expand our dried sample test offerings with ongoing plans to add new sample types. You can check out the current list of dried sample tests we offer on our sample types page. If you are interested in testing that ZRT Laboratory doesn’t currently offer, you can visit our research collaboration page to propose a dried sample test that fits your needs.