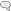The numbers are grim. It is estimated that by 2050, 13.8 million Americans will be living with Alzheimer’s disease of which over 9 million will be women (1). Alzheimer’s is the most common form of dementia and is the only disease of the nation’s 10 most common causes of death that has no highly effective pharmaceutical treatment. Alzheimer’s progresses slowly over years, robbing its victims of everything that makes them who they are – their memories, their independence, a feeling of love and connection to family and friends, even basic language for communication. In their moments of awareness, they can feel themselves and all that they are, slipping from their grasp. It is a very slow death and extremely difficult to endure as the victim and for those who love them.
Though both men and women develop Alzheimer’s, it is diagnosed in women twice as much as it is in men. In this brief article, we take a closer look at what Alzheimer’s is and the unique factors that tend to predispose women to the disease with greater frequency than men. Some of these factors include brain structure and function, effect of stress and cortisol on the female brain, and the influence of sex hormones over a woman’s lifetime.
What is Alzheimer’s disease?
Alzheimer’s is a progressive neurodegenerative disease marked clinically by dementia and pathologically by the development of neurofibrillary tangles (NFTs), beta-amyloid plaque deposition, excessive neural pruning, synapse loss, and eventual neuronal death (2, 3). Neural pruning is a normal process in which the brain removes unnecessary connections between neurons to make the brain more efficient. However, in Alzheimer’s, this process has gotten out of control and is often perpetuated by inflammation, suboptimal levels of nutrients and antioxidants, vascular conditions, reduced hormones, and neurotransmitters that support synaptic communication, and toxic exposures (4).
Characteristic of Alzheimer’s is the presence of beta-amyloid plaques and NFTs. Beta-amyloid plaques arise from the improper cleavage of amyloid precursor protein, resulting in the formation of three distinct proteins, one of which is beta-amyloid that forms plaques and fibrils. Beta-amyloid provides an anti-trophic (anti-growth) signal that promotes neuronal death. Beta-amyloid plaques may accumulate for up to 10 years prior to any discernable signs of Alzheimer’s (1, 4).
NFTs arise from the hyperphosphorylation of tau protein. Tau is found predominantly in neurons and stabilizes the internal microtubules that are part of the cytoskeleton of each neuronal cell. Microtubules are involved in mitosis (cell division) and function as tracks for intracellular transport of nutrients. When tau is hyperphosphorylated, it is removed from the microtubule, causing the microtubule to collapse. This results in disruption of several cellular processes including protein transport and cellular morphology. As tau is removed from the microtubules, it forms aggregates that lead to the development of NFTs, loss of neuronal function, and ultimately neuronal death. The overall NFT load is associated with the degree of cognitive decline (1).
The causes of hyperphosphorylation of tau protein have not been fully elucidated. It is suspected that key enzymes, that either mediate or inhibit the process of phosphorylation are upregulated and downregulated, respectively. Some of these mechanisms may be mediated by impaired glucose uptake and metabolism, which is a well-established cause of neurodegeneration (5). Additional core pathologies in Alzheimer’s include gliosis, brain atrophy with accompanying inflammation, synaptic alterations, and neurovascular breakdown (3).
Clinically, cognitive changes can begin in one’s 40s as subjective cognitive impairment (SCI). SCI is typically noticed by the individual, but standard neuropsychological testing is normal. Mild cognitive impairment typically follows SCI with standard neuropsychological testing showing the beginning of cognitive impairment. Alzheimer’s does not always follow these memory changes, but it is usually preceded by it (1).
Women and Alzheimer’s
The majority of those who suffer from Alzheimer’s are women, but this is only partially due to the fact that women tend to live longer than men. Although older age is the greatest risk factor for developing Alzheimer’s, there are distinct biological mechanisms that increase the risk and progression of Alzheimer’s in women. These risk factors include differences in brain structure and function, differences in the immune system, greater tendency to depression and sleep disorders, psychosocial stress responses, and hormonal changes over a woman’s lifetime (3). Also included in those risk factors are genetics, inflammation, and vascular issues, which can also be found in males with Alzheimer’s but develop in women through mechanisms that are unique to being female.
Female vs. Male: Brain structure and Function
Across the literature, it is agreed that men typically have larger brains than women, which may be due in part to the fact that many men are physically larger than women. Men start with larger brain volume than women and tend to have less or slower structural loss in Alzheimer’s. This has been confirmed by numerous brain imaging studies showing that annual atrophy rates were slower in male Alzheimer’s patients when compared to females (3).
Women tend to have greater cortical thickness (width of gray matter), which provides a measure of protection as it is associated with general intelligence (6). However, women also tend to have a higher rate of pathophysiological changes associated with mitochondrial function and increased oxidative stress that can lead to more rapid neurodegeneration once Alzheimer’s begins. Women also experience greater changes in the white matter of the brain that is responsible for connecting the different areas of the brain and organizing communication and information. In contrast to males with Alzheimer’s, women show a significant association between loss of white matter and lower cognition (3).
When assessing brain structure and function over a lifetime, socioeconomic status related to education, work outside of the home, income level, and engagement in activities that stimulate the brain to learn, adapt, and develop resilience are also important factors to consider. As noted by Calvo and Einstein, resilience mechanisms that prevent neuron loss include verbal processing in which the ability to produce complex, linguistically dense communication early in life seems to reduce the chance of late-life Alzheimer’s (7).
Women and the Stress Response
Adverse life events that potentiate the release of cortisol can lead to brain changes in structure and function that may increase the risk of developing post-traumatic stress disorder, depression and other neuropsychiatric disorders that might predispose women to higher rates of Alzheimer’s (3). Increased stress hormones, specifically cortisol, have been associated with cognitive impairment and Alzheimer’s. The level of corticotrophin-releasing factor 1 (CRF1) was found to be elevated in the hippocampal brain region in people with Alzheimer’s. The hippocampus is the area of the brain that allows us to form new memories. Levels of cortisol in women with Alzheimer’s were found to be much higher than in men. Increased CRF1 signaling is linked to an increase in the Alzheimer’s core pathologies of amyloidosis and tauopathy and women tend have a greater output of CRF1 in response to stress (2, 3). Differing biochemical responses to stress between men and women may reveal one of the intrinsic, sex-dependent risk factors women have for Alzheimer’s.
Stress elicits a very quick response in the brain where activation of the amygdala, hypothalamus, and the brain stem increase dopaminergic and adrenergic activity that alters the function of the prefrontal cortex. Activation of the sympathetic nervous system promotes the release of epinephrine (adrenaline) and norepinephrine (noradrenaline) from the adrenal medulla. Subsequent stimulation of the hypothalamic-pituitary-adrenal axis results in the release of cortisol. Cortisol crosses the blood-brain-barrier where it binds to receptors in the hippocampus, amygdala, and prefrontal cortex (8).
In an acute stress response, this process is normal and returns to a homeostatic state once the stressor has resolved; however, chronic activation of this process can result in the production of pro-inflammatory cytokines that influence neural activity in the brain, resulting in a reduction in synaptic plasticity and impaired memory and learning (2, 8). In human models, both men and women tend to have similar levels of peak cortisol during a stressful event, but the duration of elevated cortisol is longer in women. The effects of cortisol in response to a stressor may be relative to a woman’s level of estrogen. It is suspected that estrogen can act as a buffer against high cortisol, which might explain why symptoms of Alzheimer’s worsen or appear in menopause (2).
Through the process of aging, glucocorticoid (GC) receptors become less responsive, and the production of cortisol-binding globulin may decrease as sex hormones decrease, allowing for a higher degree of circulating free cortisol. This free cortisol can alter the normal circadian rhythm, resulting in disrupted sleep and perpetuation of the stress response with higher cortisol output (8). In both mouse and human models, beta amyloid increased in response to behavioral stressors. Stress also tends to increase the affinity of GC receptors for cortisol in the hippocampus, resulting in a reduction of hippocampal volume (2).
Estrogen Loss and Alzheimer’s disease
Sex differences in Alzheimer’s are significantly linked to the effects of estrogen. As women enter perimenopause and menopause, they can start to experience issues with short-term memory, executive function, and verbal decline. While these symptoms are often transient during the menopause transition, there is also strong correlation between Alzheimer’s development and the loss of steroid hormones as women age (7).
Neurons and glial cells are influenced by estrogen, progesterone, and androgens. More specifically, 17-beta-estradiol is protective against the development of Alzheimer’s due to its effects on neuronal and glial plasticity in women. Glial cells maintain a healthy environment to support neuronal function (7).
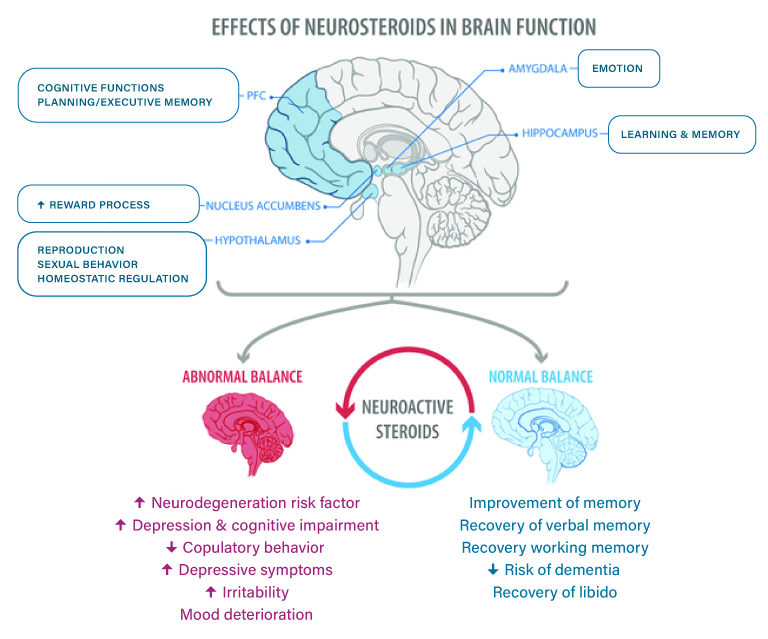




The figure shows areas of the brain regulated by steroid hormones (Top), and some of the effects found when a normal or abnormal balance between estrogen and progesterone is present (Bottom) PFC, prefrontal cortex.
In rodent studies where males were deprived of testosterone and females were deprived of estradiol, the female rodents showed a greater loss of neuronal density (7). It appears that the effects of estradiol have a uniquely important role in the female brain as compared to testosterone in the male brain. It is also important to note that the decline in estrogen during menopause occurs more dramatically and over a shorter period of time when compared to the decline of testosterone in men.
Both men and women benefit from the neuroprotective effects of estrogen, but men may maintain higher brain levels of estrogen later in life from aromatase conversion of androgens to estrogen. In both sexes, a major site of extra-gonadal estrogen synthesis is in the brain through aromatase activity. In men, in whom circulating estrogen levels are low, aromatase-dependent production of estrogens from androgens is the main source of estrogens in the brain. This is not true in premenopausal women, in whom brain levels of estrogen derive from local production as well as peripheral estrogens produced in the ovary, which diffuse freely into the brain (9). Menopausal women have low peripheral production of estrogen and lower circulating testosterone levels than men, which limits their ability to make a significant amount of estrogen in the brain through aromatase activity.
Steroid hormones play an essential role in neuronal excitability that contributes to neuroplasticity. In women, estradiol shapes memory circuits by promoting hippocampal activity. Estradiol may enhance dopamine activity, neurogenesis, and cell proliferation, while decreasing cell death and inflammation throughout the brain and nervous system. Reduced tendency towards inflammation adds to the resiliency against the development of Alzheimer’s (7).
Estradiol loss during menopause also leads to reduced mitochondrial function and neurovascular dysfunction both of which may lead to cognitive impairment. Reduced energy production in the brain and decreased blood flow can ultimately lead to cognitive decline. Neurovascular function is influenced by gonadal hormones with estradiol having the greatest influence on blood flow to the brain (7).
Estrogen Replacement Therapy
Calvo and Einstein from the University of Toronto conducted a literature review to explore the steroidal hormone mechanisms that may underlie risk and resilience in women as they age. Their goal was to determine if steroid hormones had a positive or negative influence on the development of Alzheimer’s and the impact steroid hormones would have on glial cells, neuroplasticity, brain reserve, verbal cognitive reserve, and linguistic expression (7).
Calvo and Einstein confirmed the benefits of the postmenopausal use of estradiol and concluded that it significantly reduced the risk of Alzheimer’s. They also make the distinction between the use of conjugated equine estrogens and estrogen (estradiol) replacement therapy and note that there are still questions comparing the benefits of one over the other.
Calvo and Einstein also acknowledge the potential benefits of progesterone and testosterone in the regulation of brain-derived neurotrophic factor, which influences the survival, growth, and maintenance of neurons. It was also noted that timing is key when starting hormone replacement therapy (HRT). The sooner HRT is started, the greater the benefits for preventing Alzheimer’s markers (7).
There is an optimal window of time in which the use of HRT can have its most beneficial effects. This is due to the “healthy cell bias” hypothesis that suggests that neuronal cells need to be healthy to respond positively to HRT. Once the neuronal cells have degraded, the response to HRT can be neutral or even negative (3).
Gathering Information and Starting Early
As women, we will never be without stress, and we cannot avoid the inevitable march towards menopause. We can, however, address some of the modifiable risk factors that specifically predispose women to the development of Alzheimer’s. Monitoring sex hormone levels and measuring salivary cortisol is a good start. Getting baseline values at key points in a woman’s life can guide her in making decisions as she moves through the process of hormonally transitioning from pre-menopause to menopause.
Understanding the source of our stressors and understanding the physiological influence of stress hormones on brain health is something we can all do early in life. Seeing the effects of stress on measured cortisol levels can motivate us to make the necessary changes before these habits become too deeply ingrained and have lasting effects on the health of the brain and body.
ZRT offers several testing options to measure sex hormones and salivary cortisol through all phases of a woman’s hormonal life. Cortisol can be easily measured in multi-point salivary testing taken at timed intervals throughout the day. Sex hormones can be measured in saliva, dried bloodspot, or dried urine. As a woman ages, assessing metabolic markers of HbA1c, fasting insulin, hsCRP and a complete lipid profile can provide additional information that guides dietary and lifestyle choices that aid in the prevention of glucose management issues and cardiovascular disease.
References
- Kinney JW., Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement (NY). 2018(4):575-590.
- Yan Y, Dominguez S, Fisher DW, et al. Sex differences in chronic stress responses and Alzheimer’s disease. Neurobiol Stress. 2018(8):120-126.
- Zhu D, Montagne A, Zhao Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol Life Sci. 2021;78(11):4907-4920.
- Bredesen DE. Reversal of cognitive decline: a novel therapeutic program. Aging (Albany NY). 2014;6(9): 707-717.
- Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008;15(23):2321-2328.
- Menary K, Collins PF, Porter JN, et al. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41(5):597-606.
- Calvo N, Einstein G. Steroid hormones: risk and resilience in women’s Alzheimer disease. Front Aging Neurosci. 2023;15:1159435.
- Ávila-Villanueva M, Gómez-Ramírez J, Maestú F, et al. The role of chronic stress as a trigger for the Alzheimer disease continuum. Front Aging Neurosci. 2020;12:561504.
- Alia-Klein N, Preston-Campbell RN, Kim SW, et al. Human cognitive ability is modulated by aromatase availability in the brain in a sex-specific manner. Front Neurosci. 2020;14:565668.