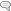At the most fundamental level, the beneficial actions of iodine derive from its ability to function as both an antioxidant and an oxidant. These basic qualities also support its effects as an antimicrobial, anti-proliferative and anti-cancer agent. How iodine functions within the human body is determined by its form, the tissue in which it resides and the overall physiological context. Iodine’s role as an antioxidant is determined by its ability to donate electrons and quench free radicals thereby reducing tissue damage and oxidative stress that may lead to chronic disease. As an oxidant, iodine can support the immune system by effectively supporting antimicrobial activity. In the presence of cancer, iodine can trigger mechanisms that are antiproliferative, promote cellular differentiation and induce apoptosis.
Iodine as an antioxidant
Free radicals are generated from both endogenous and exogenous sources. Immune cell activation, inflammation, ischemia, infection, cancer, excessive exercise, mental stress, and aging are all responsible for endogenous free radical production. Free radical production from exogenous sources results from exposure to environmental pollutants, heavy metals, certain medications, chemical solvents, certain cooking methods, cigarette smoke, alcohol, and radiation (1).
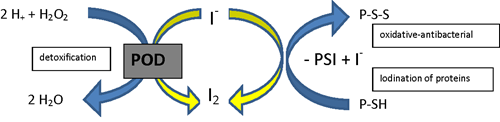
Fig 1. Iodine as a cofactor in peroxidase reactions (according to Thomas and Aune).
P-S-S = protein disulphide, PSI = iodinated protein, PSH = protein with SH-group.
Credit: Winkler R. Iodine—a potential antioxidant and the role of iodine/iodide
in health and disease. Natural Science. 2015;7: 548-557.
Antioxidants inhibit cellular damage caused by oxidative stress. Iodide functions as an antioxidant through its action as an electron donor when it interacts with peroxidase enzymes (2). In its role as an electron donor, iodide can neutralize reactive oxygen species (ROS) and prevent lipid peroxidation of cell membranes. The formation of iodolipids through the interaction of iodide with the double bonds of unsaturated fatty acids found in cell membranes, makes them less reactive to ROS. In an experiment comparing the antioxidant capacity of iodine with ascorbic acid (vitamin C) and potassium iodide, molecular iodine (I₂) was 10 times more potent than ascorbic acid and 50 times more potent than potassium iodide (KI) (3).
While the body has several endogenous systems to address oxidative stress, mineral antioxidants play an important role in electron transfer and in redox chemical reactions in the tissues where they concentrate. Overall, iodine has been shown to have a favorable impact on total serum antioxidant status (3).
Iodine as a pro-oxidant
The oxidative properties of iodine are also well documented and active within immune cells. Myeloperoxidase enzymes in leukocytes use iodine to produce iodine-free radicals that act as potent oxidants with strong bactericidal activity (4). Following application of povidone iodine (PVP-1), elemental iodine can take several forms in aqueous solution, with molecular iodine (I₂) and hypoiodous acid (HOI) having the highest antimicrobial activity. Iodine molecules oxidize vital pathogen structures including amino acids, nucleic acids and membrane components. The action of iodine disrupts microbial cell walls by inducing pore formation, leading to cytosol leakage with eventual destruction of the pathogen (5).
Iodine and the immune system
Iodine is taken up and metabolized by immune cells where its function as either an anti-inflammatory or proinflammatory agent is determined by the physiological context. Iodine can support the innate immune system to fight bacterial and viral infection and has immunomodulatory effects on immune cells. This immune enhancing effect increases expression of cytokines and chemokines that control cell trafficking and regulate the nature of the immune response. Overall, this has the effect of enhancing the immune system’s ability to fight infection while keeping the immune response balanced (6).
Phagocytes, granulocytes and monocytes, types of leukocytes cells within the immune system, harbor the highest concentration of iodide transporters. In vitro studies show that iodine induces transcriptional modification in human leukocytes, resulting in the upregulation of genes that promote activation of cytokines and chemokines. The observed transcriptional changes in the leukocytes produced a mix of cytokines that were both pro- and anti-inflammatory, indicating a balanced immune response that can both promote and resolve inflammation (7).
Respiratory infections
It is well established that iodine has a broad spectrum of antimicrobial activity against bacterial, viral, fungal, and protozoal pathogens and has been used as an antiseptic for the prevention of wound infections for several decades. A 2021 article in Ear, Nose & Throat Journal, published an in vitro study by Pelletier et al establishing that nasal and oral povidone iodine (PVP-1) solutions are effective at inactivating SARS-CoV-2 at a variety of concentrations after 60-second exposure times. They concluded that the formulations tested may help to reduce the transmission of SARS-CoV-2 if used for nasal decontamination, oral decontamination, or surface decontamination in known or suspected cases of COVID-19 (8).
Pelletier’s study was duplicated using a different iodine formula known as Essential Iodine Drops (EID), which is a derivative of fulvic acid complexed with molecular iodine (I₂) at a concentration of 200 mcg/mL. The SARS-CoV-2 virus was exposed to EID in vitro for 60 and 90 seconds and in both cases, the viral load decreased by 99% (9).
Nasal goblet and ciliated cells within the respiratory epithelium have the highest expression of angiotensin-converting enzyme 2 (ACE2), the main receptor for COVID-19. The mechanism of action of PVP-I and EID as a mouth rinse and nasal spray targets the ACE2 receptor for inhibition. It diminishes the ACE2 receptors in the lymphocytes of the mucosal tissue, reducing the concentration of SARS-CoV-2 shed into saliva and nasal fluid (9).
Although 10% PVP-I solutions had been previously tested against human coronaviruses SARS-1 and MERS, these solutions are unsuitable for use in the nasal and oral cavities at commercially available concentrations. Diluting this 10% solution to a 1% solution allows for use of PVP-I in the nasal and oral cavities. Solutions as low as 0.5% PVP-I have been found to be effective at appreciably reducing SARS-CoV-2 in vitro (9).
The use of oral potassium iodide (KI) in the treatment of respiratory syncytial virus (RSV) was studied in lambs, as they respond similarly to this virus as infants. As mentioned above, iodine supports oxidative pathways as part of an effective immune response. Iodide can be concentrated in the nasal mucosa after oral iodide administration and integrates into the oxidative system that generates hypoiodous acid (HOI), which has potent microbicidal activity against bacteria and viruses, including RSV. Overall findings indicate that the use of high-dose potassium iodide (1.7 mg/kg body weight) in vivo lessens the severity of RSV infections through augmentation of the mucosal oxidative defenses (10).
Anti-cancer effects of iodine
In addition to functioning as an antioxidant, anti-inflammatory, and antiproliferative agent, iodine also has the ability to induce apoptosis and differentiation in cancer cells. The reactive oxygen species of singlet oxygen, superoxide anions, hydrogen peroxide and hydroxyl radials have a wide range of cellular and molecular effects, resulting in mutagenicity, cytotoxicity, and alterations in gene expression. Molecular iodine (I₂) can function as a scavenger and antioxidant that neutralizes various ROS that are known to be cytotoxic and implicated in the development of cancer (3).
Molecular iodine (I₂) inhibits cell proliferation and induces apoptosis in cancer cells through a direct mitochondrial effect and an indirect effect through iodolipid generation. Through its oxidant/antioxidant properties, molecular iodine (I₂) can directly dissipate the mitochondrial membrane potential, triggering mitochondrion-mediated apoptosis in cancer cells without affecting the mitochondria of normal tissue (3).
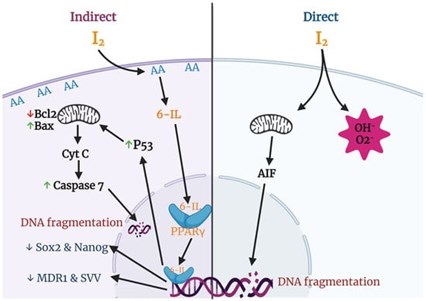
Fig 2. Anti-cancer effects of iodine associated with mitochondria and PPAR gamma.
Credit: Aceves C, Mendieta I, Anguiano B, et al. Molecular iodine has extrathyroidal effects as an
antioxidant, differentiator, and immunomodulator. Int J Mol Sci. 2021; 22(3):1228.
In the presence of high levels of arachidonic acid (AA), molecular iodine (I₂) induces the formation of 6-iodolactone (6-IL), which is an iodinated derivative of AA. AA is a polyunsaturated free fatty acid present in the membrane phospholipid layer of all mammalian cells. Tumors contain a significantly higher concentration of AA, and when treated with molecular iodine (I₂), 6-IL greatly increases. It is proposed that the increase in 6-IL indirectly contributes to the antiproliferative and apoptotic effect of molecular iodine (I₂) (3, 11).
Additionally, 6-IL has a high affinity for peroxisome proliferator-activated receptor gamma (PPARγ). PPARs are nuclear transcription factors that regulate cancer cell proliferation in addition to their classical role in maintaining lipid and glucose homeostasis (11). PPARs exist as three substrates, with PPARγ having the highest affinity for IL-6. AA is a natural ligand of PPARs, meaning that it binds readily to this receptor. When molecular iodine (I₂) promotes the formation of 6-IL in the presence of AA, 6-IL will bind to PPARγ with an affinity six times higher than AA. The binding of 6-IL to PPARγ results in a regulating effect on cancer cell proliferation (11). In a preliminary clinical study of 22 women with breast cancer, those who received 5 mg/day of molecular iodine (I₂) rather than placebo showed an increase in PPARγ expression, increased apoptosis, and decreased proliferation of cancer cells (12).
Iodine and breast health
Iodine concentrates in many extrathyroidal tissues and has particular anti-tumorigenesis effect in mammary, prostate, pancreas, lung, and nervous system tissue. These tissues exhibit the specific ability to take up molecular iodine (I₂) and promote apoptosis through the induction of PPARγ. Molecular iodine (I₂) and 6-IL have been studied in the treatment of several types of tumor cell lines, showing a suppressive effect on the development and size of both benign and cancerous neoplasias (3, 13).
Molecular iodine's (I₂’s) role in maintaining breast health is evident in its ability to effectively address cyclic mastalgia from fibrocystic breast disease. Both in vitro and in vivo studies of mammary cancer have shown that molecular iodine (I₂) treatment induces apoptosis and increases expression of sodium-iodide symporter (NIS) and pendrin (iodine receptors), to increase the uptake of iodine. Molecular iodine (I₂) also promoted a reduction in metastatic inducers such as vascular endothelial growth factor that, in this context, increases blood flow to cancerous tissues. It is suspected that the trigger for these events is through the activation of PPARγ which, as mentioned above, is apoptotic, antiproliferative, and promotes differentiation (3).
In a study presented by Aceves et al, 0.05% molecular iodine (I₂) solution prevented the induction of DNA adduct formation in premalignant cancer tissues in the presence of dimethylbenz(α)anthracene, a potent carcinogen. DNA adducts can initiate and promote cancer and tend to be present in high amounts in the urine of breast cancer patients and women at high risk for breast cancer. Breast cancer tissue also contains a higher concentration of AA. After treatment with 0.05% molecular iodine (I₂), 6-IL levels increased 15-fold higher than in normal mammary tissue, promoting apoptosis and reducing proliferation by increasing the activity of PPARγ (3).
Iodine and prostate health
In prostate tissue, iodine has proven useful in both benign and cancerous conditions. Japanese men have much lower rates of prostate cancer than men in the United States. The Japanese diet is notably high in iodine as compared to an American diet with an estimated consumption over 20 times that of people in the United States. As reported by Tina Kaczor, ND, in Natural Medicine Journal, animal studies using 0.05% molecular iodine (I₂) supplementation reduced symptoms of benign prostatic hyperplasia (BPH). It was also noted that 5 mg per day of Lugol’s solution improved urine flow and reduced prostate-specific antigen values over an eight-month period (14).
Iodine’s effects in prostate cancer are noted by Navarra in a 2010 issue of Urology where the NIS was found in 52% of prostate adenocarcinomas and was associated with an increased aggressiveness of the tumor (15). The presence of NIS in the tumors can be an indication of iodine deficiency in the prostate tissue, reducing its ability to prevent cellular changes associated with the tumorigenesis.
The dose response effect of iodine in benign and cancerous conditions
Molecular iodine (I₂) is the form of iodine heralded for its antineoplastic effects. Although seaweed contains iodine in several chemical forms, molecular iodine (I₂) is commonly found in seaweed consumed in traditional Asian cultures and used for treatment of breast cancer due to its ability to soften tumors and reduce nodulation (3).
Dose response studies in humans demonstrated that iodine supplemented at 1.5 mg/day or less had no effect on benign pathologies whereas, dosages of 3.5 mg/day up to 6 mg/day, mainly in the form of molecular iodine (I₂), exhibited significant beneficial actions on mastalgia and BPH. Dosages at 9 mg/day and 12 mg/day showed the same benefits but had a greater impact on thyroid function and produced a variety of minor side effects (3, 13).
In summary
I hope this three-part series has satisfied your curiosity about iodine as much as it has mine. The fact that 30% of the global population is iodine deficient is concerning especially when we consider that iodine has so many functions and concentrates in a variety of tissues. Our need for iodine may be dependent on a number of factors including where we live, what we consume and how much iodine our own body needs to stay sufficient while supporting all of the processes that depend on an adequate amount of iodine.
Testing for iodine sufficiency
ZRT Laboratory measures iodine through a dried urine sample taken upon waking and before bed. This test does not require a high-loading dose of iodine prior to collecting the urine sample and is more representative of daily consumption. The options for testing include a singular Iodine Panel, or a measurement of iodine in the Urine Toxic & Essential Elements add-on profile and the Comprehensive Thyroid Panel, which is a combination of the Elite Thyroid Panel and the add-on Urine Toxic & Essential Elements.
References
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763.
- Venturi S, Venturi M. Evolution of dietary antioxidants: role of iodine. Lecture held at the “Thyroid Club” Annual Meeting of Bologna University. February 2007.
- Aceves C, Mendieta I, Anguiano B, et al. Molecular iodine has extrathyroidal effects as an antioxidant, differentiator, and immunomodulator. Int J Mol Sci. 2021;22(3):1228.
- Karbownik-Lewińska, M Stepniak J, Iwan P, et al. Iodine as a potential endocrine disruptor—a role of oxidative stress.” Endocrine. 2022;(78)2:219-240.
- Eggers M. Infectious disease management and control with povidone podine. Infect Dis Ther. 2019;(8)4:581-593.
- Mohamad RA. Iodine, an effective substance against the COVID-19 pandemic. Anim Husb Dairy Vet Sci. 2021;(5)2:2.
- Bilal MY, Dambaeva S, Kwak-Kim J, et al. A role for iodide and thyroglobulin in modulating the function of human immune cells. Front Immunol. 2017;(8):1573.
- Pelletier JS, Tessema B, Frank S, et al. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Ear, Nose Throat J. 2021;100(2_suppl):192S-196S.
- Köntös Z. Efficacy of "essential iodine drops" against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). PLoS One. 2021; Jul 9;16(7):e0254341.
- Derscheid RJ, van Geelen A, Berkebile AR, et al. Increased concentration of iodide in airway secretions is associated with reduced respiratory syncytial virus disease severity. Am J Respir Cell Mol Biol. 2014;(50)2:389-397.
- Nava-Villalba M, Nuñez-Anita RE, Bontempo A, et al. Activation of peroxisome proliferator-activated receptor gamma is crucial for antitumoral effects of 6-iodolactone. Mol Cancer. 2015;14:168.
- Vega-Riveroll L, Mondragon P, Rojas-Aguirre J, et al. Impaired nuclear translocation of estrogen receptor alfa could be associated with the antineoplastic effect of iodine in premenopausal breast cancer. Cancer Res. 2011;70(24 Supplement):P6-14-15.
- Aceves C, Anguiano B, Delgado G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. 2013;23(8):938-946.
- Kaczor T. Iodine and cancer: a summary of the evidence to date. Nat Med J. 2014;6(6).
- Navarra M, Micali S, Lepore SM, et al. Expression of the sodium/iodide symporter in human prostate adenocarcinoma. 2020;(75)4:773-778.