Practice Takeaway: Providers should be aware that three different body fluids – saliva, blood or urine – can be used to assess adrenal gland function, and should know each method’s advantages and disadvantages, when deciding how to test patients.
Inadequate or excessive production or disrupted circadian patterns of cortisol synthesis by the adrenal glands in response to stressors can eventually lead to imbalances in blood glucose levels, impaired immune response, as well as a host of different hormonal imbalances, all of which are associated with multiple adverse conditions and symptoms.
Saliva
Cortisol is synthesized by the adrenal glands and released into the bloodstream in response to stress signals from the brain. In the bloodstream cortisol is mostly bound up by Cortisol Binding Globulin (CBG) and albumin, leaving only about 1-3% bioavailable to enter tissues and invoke a biological response. The bioavailability of cortisol can vary considerably due to different levels of CBG; liver synthesis of CBG is controlled by various hormones, such as estrogens, thyroid, and cortisol itself. Salivary cortisol reflects the amount of cortisol that escapes binding proteins, and enters the tissues throughout the body, including the salivary glands and saliva.
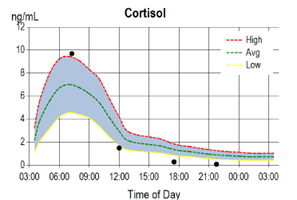
As such, saliva is representative of the bioavailability of cortisol to target tissues throughout the body. Saliva collection at four time points throughout the day (morning, noon, evening, and night) provides a simple and convenient means for assessing not only the bioavailability of cortisol but also its circadian pattern of synthesis (i.e. the diurnal cortisol curve).
Blood
Blood serum and plasma derived from venipuncture, as well as whole capillary blood derived from the fingertip and dried on filter paper (dried blood spot, DBS) have been used to determine total cortisol levels in the bloodstream. Cortisol levels in plasma, serum, and DBS are quantitatively equivalent.
DBS testing for cortisol does have the advantage over conventional venipuncture serum/plasma methods in that the former is more convenient for the patient since blood can be collected at any time and place, and allows for morning and night collections outside the clinical setting.
While cortisol testing in blood provides a good estimate of the adrenal glands’ capacity for total cortisol synthesis and release into the circulation, this test is not useful for determining the bioavailable fraction of cortisol without additional testing for CBG, which is not commercially available.
Urine
Urine represents another easily accessible body fluid for measuring cortisol. Urine cortisol is tested commonly in most laboratories by liquid chromatography-mass spectrometry (LC-MS) using urine collected in a large plastic container over a 24 hr time course. More recently, simple and convenient methods have been developed to measure cortisol from urine collected at four times during the day (first morning, late morning, evening, and night before bed) and dried on filter paper.
Steroid hormones like cortisol, whether from urine or blood, are extremely stable at room temperature for up to a month when dried on filter paper, allowing for great latitude in the dried urine assay. The 4x dried urine method has the advantages of ease of sample collection (urination directly on a filter card and hang to dry) and shipment (dry filter card vs liquid urine).
. . . the 4x dried urine cortisol curve, like the 4x saliva cortisol curve, conveys important clinical insight into the adrenal glands' response to stressors throughout the day. |
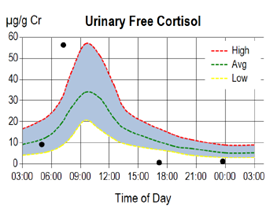
Moreover, by collecting urine 4x from morning to night it is possible to evaluate the circadian rhythm of cortisol synthesis, as opposed to total cortisol synthesis over a 24 hr period by the liquid urine method. The circadian patterns of cortisol synthesis can vary considerably among individuals and thus the 4x dried urine cortisol pattern, like the 4x salivary cortisol assay, will convey important clinical insight into the adrenal glands' response to stressors throughout the day.
Cortisol is present in urine at about equal concentrations in both a conjugated (mostly glucuronidated) and free form. The free form, designated Urinary Free Cortisol (UFC), enters urine in a way similar to salivary cortisol and is representative of the bioavailable fraction.
Interpretation of the 4x circadian UFC is somewhat different from saliva, since the first urine void is representative of cortisol synthesis throughout the night and is expected to be lower than the second urine void, which, like the first saliva collection, should represent the peak of adrenal cortisol synthesis in the early morning. In a healthy individual cortisol in both saliva and urine tapers as the day progresses and reaches a nadir before bed at night. Circadian patterns of adrenal cortisol synthesis (i.e. diurnal cortisol curves) are usually very similar when measured in saliva or urine.
The diagrams below depict typical circadian patterns of salivary and urinary free cortisol synthesis in an individual showing adrenal fatigue as the day progresses. As seen in the diagrams, both the salivary and UFC show normal circadian patterns, but low cortisol synthesis in the afternoon and at night before bed, indicating an inability of the adrenal glands to maintain normal cortisol synthesis by day's end.
While the 24 hr total or free cortisol levels are usually within normal range in these individuals, their circadian patterns reveal abnormally low late evening/night free urinary cortisol levels which commonly parallel their symptom profile of fatigue occurring more at night than in the morning.
In summary, providers should be aware that three different body fluids can be used to assess adrenal gland function, and should know each method’s advantages and disadvantages, when deciding how to test patients. Working with a lab that offers the clinical expertise to interpret the differences between these methods also helps ensure patients get the most meaningful results.
Related Resources