Sometimes, when we endeavor to understand and describe complicated medical topics, there is a temptation to find a simple explanation to cut through the complexity. These explanations can help bridge the knowledge gap for a while, but as our knowledge grows, they lose some of their original usefulness (e.g., the notion of “good” and “bad” cholesterol).
In some cases, those over-simplified explanations actually become a hindrance to helping clinicians and patients understand the important mechanisms and solutions related to chronic conditions. The use of terms like “adrenal fatigue” and “adrenal exhaustion” to summarize the complex dysfunctions related to the stress response is one such explanation. Though these terms have helped dispel the notion that only extreme issues related to adrenal function (Addison’s disease or Cushing’s disease) are of clinical importance, and have become surrogate descriptions for stress-related outcomes, they should now be replaced by more accurate and medically appropriate terms, like HPA axis dysfunction, adrenal insufficiency, or where applicable, hypocortisolism.
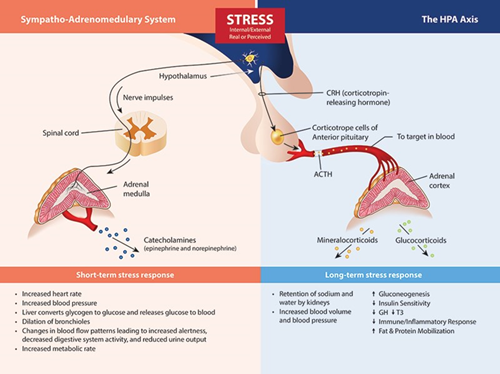
The Stress Response System(s). Beginning in the brain, stress signals are communicated by direct innervation to the adrenal medulla to cause a nearly immediate release of catecholamine’s (the fight or flight response) and through neuro-hormone signals within the HPA axis that influence the release of cortisol, DHEA(S) and aldosterone.
Does Nomenclature Reassessment Matter?
While it is true that the most common laboratory method to assess the function of the HPA axis is through the measurement of hormones secreted by the adrenal glands, primarily cortisol and DHEA(S), the mechanisms that control the level of these hormones resides mostly outside of the adrenal gland. Low cortisol and DHEA(S) levels may indeed be related to chronic stress, but as a result of HPA axis adaption (down-regulation) to protect tissues from excess cortisol, have little to do with the inherent capability of the adrenal gland to produce these hormones (see adrenal insufficiency below). While many clinicians (and laboratories) still refer to this as “testing the adrenals,” it is much more accurate to say that such testing is assessing the status of the HPA axis using adrenal hormone measurements as surrogate markers. So, why does this nomenclature reassessment matter?
Using descriptive and accurate terms helps clinicians and patients better understand the pathophysiology caused by stress and the stress response system. |
First of all, using descriptive and accurate terms helps clinicians and patients better understand the pathophysiology caused by stress and the stress response system. In most cases, issues related to perceived stress, glycemic control, circadian rhythm, cortisol feedback control (in the hypothalamus and/or pituitary), inflammatory signaling, or tissue-specific glucocorticoid effects will have much more to do with a treatment protocol than direct support of adrenal function.
For instance, many adaptogenic herbs and nutrients that were once thought to function primarily by supporting adrenal function have been shown to have mechanism that modulate non-adrenal HPA axis or glucocorticoid signaling functions. Related to this is the ability of the clinician to interface appropriately with the vast amount of literature that describes patient outcomes related to stress and HPA axis function. The term “adrenal fatigue” is virtually absent from the peer-reviewed literature and has even caused the Endocrine Society to warn the public against the diagnostic “myth” of adrenal fatigue and to cast suspicion upon clinicians using such terms. While I generally agree with the Endocrine Society that the term “adrenal fatigue” is problematic, I do not agree with them that there is little evidence to connect chronic stress with adverse health outcomes, or that testing adrenal hormone output is of no value beyond diagnosing extreme adrenal disease conditions.
An increasing body of research links a variety of chronic dysfunctions with specific patterns of adrenal hormone output (basal or provoked). By avoiding the use of oversimplified (and incorrect) terminology to describe these relationships and instead choosing more appropriate descriptive terms, the clinician will enhance the credibility of this important phenomenon and be better equipped to incorporate therapies that address the complexity of the whole stress response system.
What are More Appropriate Terms?
HPA Axis Dysfunction (or Maladaption): This term is much more appropriate to describe the many consequences that link stress (allostasis) with the myriad of measurable negative outcomes related to the stress response. The majority of these outcomes can be linked in some manner to processes controlled by the HPA axis. Alternatively, some refer to these as “disorders of the stress system” or the “consequences of the maladaption to stress.”
Hypocortisolism: This is the most descriptive term to use when measured cortisol is well below the laboratory reference range. Still, it is a relative term and does not necessarily implicate dysfunction or “fatigue” of the adrenal gland. Extreme hypocortisolism is associated with Addison’s disease and other forms of primary and secondary adrenal insufficiency. Reduced HPA axis function resulting in low cortisol levels is common in PTSD, fibromyalgia, chronic fatigue syndrome, certain affective disorders, and individuals with high psychosocial “burnout.” Other specific terms for different stress-related HPA axis phenomena include hypercortisolism, loss of HPA circadian function, and low circulating DHEA(S).
Adrenal Insufficiency: This is a clinical manifestation that results in a deficient production or action of glucocorticoids, a condition that has potential life-threatening consequences. Primary adrenal insufficiency (i.e., Addison’s disease) describes diseases intrinsic to the adrenal cortex primarily caused by autoimmune adrenalitis. Secondary adrenal insufficiency relates to insufficient pituitary ACTH or intrinsic defects in the adrenal responsiveness to ACTH. Tertiary adrenal insufficiency results from impaired synthesis of hypothalamic CRH or AVP. The most common cause of tertiary adrenal insufficiency is the chronic use of exogenous glucocorticoids (pharmacotherapy), leading to the suppression of hypothalamic secretions of CRH. True adrenal insufficiency will almost always require hydrocortisone replacement therapy (often life-long). For a complete review of the etiology, pathophysiology, clinical presentation, diagnosis and treatment approaches to adrenal insufficiency, see Charmandari, et al.1
1Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014 Jun 21;383(9935):2152-67.